

This time no formal charges are present - each oxygen atom needs 6 electrons and gets 6 electrons, the same being true for sulfur. Another Lewis structure that can be drawn for SO2 is this one. The charges on the atoms are '+1.4' for sulfur and '-0.7' for each oxygen atom.

This corresponds to sp2 hybridization as well. In the Lewis structure of formaldehyde, each atom should have a formal charge of zero.The formal charge is a way to determine the distribution of electrons in a molecule. The negative charge will be split on the two oxygen atoms. So, it also has three sigma bonds and no lone pairs. Lewis Structure The chemical formula CF4 represents Carbon Tetrafluoride. This corresponds to sp2 hybridization.įor the second carbon atom (CH), it forms one single bond with the first carbon atom, one single bond with the hydrogen atom, and one single bond with the oxygen atom. The formal charge of the formaldehyde (H2CO) Lewiss structure. So, it has three sigma bonds and no lone pairs. So I take half of eight and that's going to give me a formal charge of zero as well. It would have four electrons in the isolated atom. So, the formal charge of the carbon atom in H2CO is +1.įor Question 2, we need to find the hybridization of the two carbon atoms in H2C-CH-OH.įor the first carbon atom (H2C), it forms two single bonds with two hydrogen atoms and one single bond with the second carbon atom. Now, I'm going to look at my carbon atom.
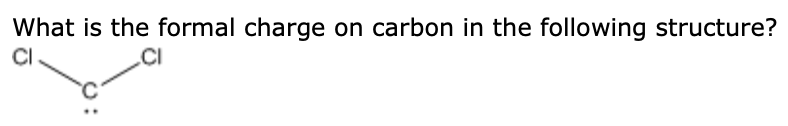
So, there are no non-bonding electrons and 6 bonding electrons (2 from each single bond and 4 from the double bond).įormal Charge of Carbon = 4 - 0 - (1/2 * 6) = 4 - 3 = 1 In H2CO, carbon forms two single bonds with two hydrogen atoms and one double bond with one oxygen atom. The formula for formal charge is:įormal Charge = (Valence Electrons) - (Non-bonding Electrons) - (1/2 * Bonding Electrons)Ĭarbon has 4 valence electrons. Which molecule contains carbon with a negative formal charge Group of answer choices. For Question 1, we need to find the formal charge of the carbon atom in H2CO (formaldehyde). Which molecule contains carbon with a negative formal charge Group of answer choices CH4 CN- CO32- CS2 19.What is the frequency of electromagnetic radiation with a wavelength of 0.25 km 17.


 0 kommentar(er)
0 kommentar(er)
